Dear Friends,
First of all, I want to express my gratitude for everything you’ve helped me achieve here. Your kind words and encouragement keep me motivated and inspired.
My three guiding principles are: Improve clinical care, Raise awareness, and Provide a cutting-edge treatment for Wolfram syndrome. Here is our progress:
A Drug-Repurposing Clinical Trial
Our drug-repurposing clinical trial of dantrolene sodium in patients with Wolfram syndrome has been almost concluded. Nineteen patients could successfully complete the required six-month phase, and many of them decided to stay on dantrolene sodium another 18 months. The results of this open-label clinical trial (all the participants took dantrolene sodium) show that dantrolene sodium is well tolerated by patients with Wolfram syndrome. Although the study was small, a select few patients seemed to have improvements in diabetes-related outcomes, which might correlate with a positive trend in other outcome measures, including visual acuity and brain functions. This study justifies further investigation into using dantrolene sodium and other new drugs targeting the same molecular pathway for the treatment of Wolfram syndrome.
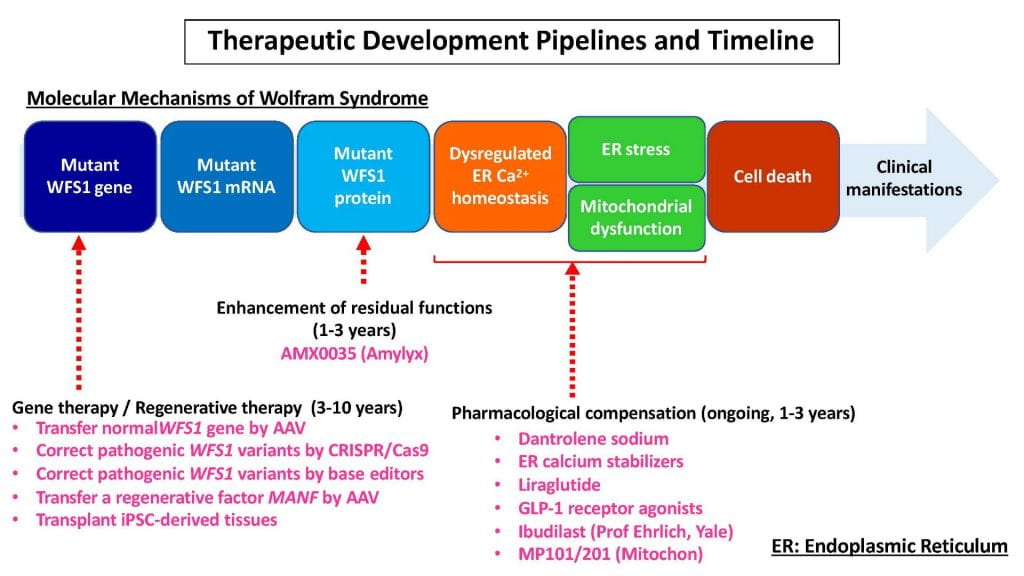
Novel Drugs
We are aware that a drug-repurposing is not the best approach to halt the progression of Wolfram syndrome. We need cutting-edge treatments designed explicitly for Wolfram syndrome. Based on the clinical trial data of dantrolene sodium in patients with Wolfram syndrome, we have been actively developing novel drugs in collaboration with the drug development team at the National Institutes of Health (NIH)/National Center for Advancing Translational Sciences in the United States and a few biotech companies. We are currently focusing our efforts on developing AMX0035 together with Amylyx in Cambridge, MA, and ibudilast together with Professor Ehrlich at Yale University. We are also starting collaboration with Mitochon Pharmaceuticals.
Regenerative Gene Therapy
My current focus is to develop gene therapy for Wolfram syndrome. Our ultimate goal is to provide a cure using regenerative gene therapy. We have been trying to improve diabetes, visual acuity, and brain functions using viral vectors of a healthy Wolfram gene and a regenerative factor called MANF in mouse models. We are getting encouraging preliminary results and have published two articles recently. We are currently testing two ways to deliver genes through intravitreal (for optic nerve) and intraventricular (for brain) injections.
Base Editing Gene Therapy
In collaboration with Dr. David Liu’s team at Harvard University/Broad Institute and Dr. Catherine Verfaillie’s team at the Katholieke Universiteit Leuven, we have been developing a novel gene therapy called Base Editing for Wolfram syndrome. This technology uses some components from CRISPR systems together with other enzymes to directly replace abnormal WFS1 gene with normal WFS1 gene. Although we are still at the early preclinical stage using cell models of Wolfram, we hope that we can bring this technology to our patients in the next 3-10 years. Please stay tuned.
New Genetics Clinic
To further improve the clinical care for patients with Wolfram syndrome and Wolfram-related disorders, I have created a new genetics clinic at Center for Advanced Medicine, Washington University Medical Center. We offer genetic evaluations, education, and counseling for patients and family members of all ages with or suspected to have Wolfram syndrome or WFS1-related disorders. We also provide personalized management plans based on the type of your gene variants together with other specialists at our medical center, such as Dr. Marshall, Dr. White, Dr. Hoekel, and beyond. To make an appointment with me, please call 314-747-7300 or 314-747-3294 (if you are participating in our research clinic/registry or interested in participating in the research). You can also send an email to WolframSyndrome@wustl.edu. We could cover the costs for genetic testing if your insurance does not cover the entire amount of the costs.
Finally, I want to express my gratitude to Dr. Hershey, Dr. Marshall, Mrs. Samantha Blankenship, Dr. White, and other physicians and scientists for running the Wolfram research clinic study, Mrs. Cris Brown and Mrs. Stacy Hurst for managing the Wolfram registry and clinical study, scientists in my lab, collaborators all over the world, including Dr. Barrett, Dr. Plaas, Dr. Terasmaa, Dr. Millman, Dr. Ehrlich, and supporters for my research. I also want to thank patient organizations in the world and private donors supporting my research, including the Silberman Fund, the Ellie White Foundation for the Rare Genetic Disorders, the Snow Foundation, the Unravel Wolfram Syndrome Fund, the Stowe Fund, the Eye Hope Foundation, the Feiock Fund, Associazione Gentian – Sindrome di Wolfram Italia, Alianza de Familias Afectadas por el Sindrome Wolfram Spain, Wolfram syndrome UK, and Association Syndrome de Wolfram France.
As always, please feel free to contact me with any questions (urano@wustl.edu). I would like to know what you think and how you feel. Thank you again for your continued support and encouragement. I have no doubt better days are ahead. We will go through this challenging period with unusual optimism and courage. We will continue working as one team and change history together.
Sincerely,
Fumi Urano, MD, PhD
September 6, 2020